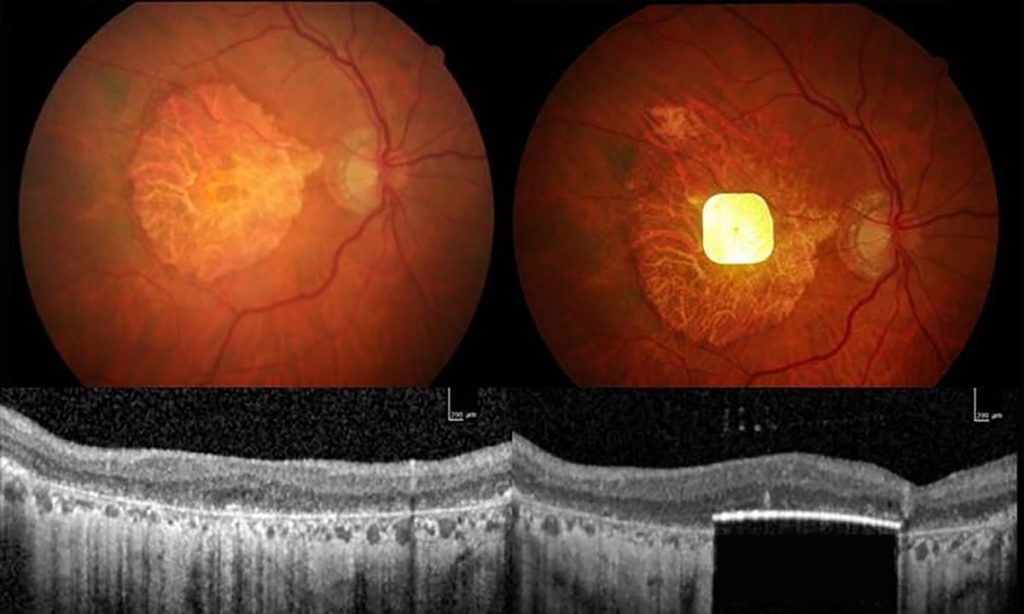
Scientists have used an eye implant to improve the vision of dozens of people left functionally blind by age-related macular degeneration (AMD). The implant, which measures 2 millimetres by 2 millimetres, and is just 30 micrometres thick, is surgically inserted beneath the retina to replace the light-sensitive cells that have been lost to the disease.
The clinical trial, which is described today in The New England Journal of Medicine, involved 38 people with advanced AMD whose retinas had degenerated severely. One year after device implantation, 80% of participants had gained a clinically meaningful improvement in their vision.
“Where this dead retina was a complete blind spot, vision was restored,” says trial leader Frank Holz, an ophthalmologist at the University of Bonn in Germany. “Patients could read letters, they could read words, and they could function in their daily life.”
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Despite some minor events related to implantation surgery, the trial’s safety-monitoring board viewed the device’s benefits as outweighing its risks. In June, the device’s owners — the San Francisco-based neurotechnology company Science Corporation — applied for certification that would allow the device to be sold on the European market.
“I think this is an exciting and significant study, which has been well-designed and analysed. It gives hope for providing vision in patients for whom this was more ‘science-fiction’ than reality,” says Francesca Cordeiro, an ophthalmologist at Imperial College London.
Restored vision
AMD is the commonest form of incurable blindness in older people. There are two main types, wet and dry AMD. The current work studied people with dry AMD, the advanced form of which affects around 5 million people globally. In dry AMD, the central retina’s light-sensitive cells die over a period of years, leaving affected individuals with intact peripheral vision but without their high-acuity central vision. “They can’t recognize faces, they can’t read, they can’t drive a car, they can’t watch television,” says Holz.
The light-sensitive cells that die (rods and cones) convert light into electrochemical signals that are conveyed to other types of retinal neurons, which then send messages to the brain’s visual-processing regions. Because retinal neurons survive AMD, scientists reasoned that a light-sensitive implant that electrically stimulates the retina according to the pattern of photons striking it could reinstate a sense of vision.
The implant, termed PRIMA — for photovoltaic retina implant microarray — was originally developed by the Paris-based company Pixium Vision, and was acquired by Science Corporation last year. It is wireless, unlike previous retinal devices. And, being photovoltaic, the photons that activate it also provide the energy source for generating its electrical output.
It is used in combination with glasses that contain a camera that captures images and converts them into patterns of infrared light that they transmit to the retinal implant.
The system, which allows users to zoom in and out on target objects, and adjust contrast and brightness, does, Holz says, take months of intensive training to use optimally.
In the current study, 38 individuals were treated at 17 clinical sites across 5 European countries, and 32 of the participants were tested a year after implantation. Twenty-six of them had a clinically meaningful improvement in their vision — which, on average, amounted to being able to see two lines further down a standard eye test chart of letters. Overall, most participants’ vision came close to the resolution achievable with PRIMA.
By the study’s end, most recipients were using PRIMA at home to read letters, words and numbers. Of the 32, 22 said that their user satisfaction was medium to high.
Slow reading
However, a questionnaire about users’ daily quality of life revealed no significant overall improvements. A retinal-degeneration researcher working on treatments for vision loss who wished to remain anonymous to avoid retaliation, spoke to Nature and raised concerns that intensive visual training and the motivation of having received an exciting medical device might have led to improved test results. They said that the results would have been more robust if gains had been demonstrated relative to a randomised placebo group that had received the glasses and training protocols but no implant.
Holz, too, acknowledges that the current system has limitations, and says he expects future implants to be more effective. “With this first major breakthrough, it’s a starting point for further improvement,” he says.
Another concern is the current maximal acuity achievable with the current device. The PRIMA system has only 381 pixels, each 100 micrometres square. And Holz concedes that users’ reading is “not fast, fluid reading”. The vision provided is also black and white not colour.
Holz says Daniel Palinker, a physicist at Stanford University in Palo Alto, California, who originally designed the device, has ideas about how to one day achieve colour vision. A next-generation device that is larger than PRIMA and filled with smaller pixels should enable better visual acuity, “It’s the beginning of a journey,” Holz says.
Although the device has been tested in people with AMD, it could also help to restore sight in people affected by other conditions in which photoreceptor cells die but other retinal neurons remain functional, such as retinitis pigmentosa.
Retinal implants are not the only approach being developed for this problem. Other investigators are exploring the use of stem-cell therapies to regenerate photoreceptors; optogenetic therapies, in which light-sensitive proteins are introduced into the remaining retinal cells; and even implants that are inserted into the brain’s visual cortex.
“It’s a huge dynamic space, and there are lots of approaches now,” says Holz. “Which will pan out in the end, nobody knows.”
This article is reproduced with permission and was first published on October 20, 2025.